Your Cart is Empty

Vacuum Thermodynamics in Our Freeze-Dried Universe
Freeze dry systems are some of the more thermodynamically active vacuum applications around, with operating pressure requirements firmly categorized in the “medium vacuum” realm. The lessons learned from the principles and subsystems in play can and should be applied broadly; to your own industrial vacuum system whether freeze-drying is your game or not; and, just maybe, in appreciation of the happenstance of our own humanity.
Written by: Bryan A. Jensen
Table of Contents
- The Sublimation of Ice Water
- Observing Hot Ice in a Vacuum
- Clouds: Earth's Coma
- Vacuum Chamber to Pump
- Vacuum Systems and Condensable Vapor load
- Lessons in Freeze Drying
Leaving a waffled imprint in the recently laid taupe carpet in our bedroom, I carefully placed my burgundy moonboot on the splintered wooden footboard of my brother’s lower bunk. The oversized brown & golden lion staring up at me in the moonlight menacingly contorted its eyebrow, driven by my own personal Buzz McCallister’s shoulder rolling over underneath his blanket. I froze; desperately not wanting to wake the slumbering beast.
We had one of those L-shaped bunk sets, not the traditional stacked parallel style, and one midnight twilight many months before I’d been very Kevin-ly dangling my hand down over the upper bunk’s safety rail in a game of cat and mouse while bro feigned an attempt at sleep.
On that night, the mouse’s arm got caught two-handed at wrist & elbow and before I knew it, I was heels over head, somersaulting down on top of him while the former accelerated into the board, right where the griddled sole of my same foot’s boot now quietly rested.
Breathing slowly, watching for movement and after an eternity, perhaps 23 seconds, I decided to make my move. A quick light step where the lion’s cheek met its mane would avoid stomping my brother’s stomach and the subsequent brought fury. But as I stepped up into action, the splintering suddenly completed under foot. I flailed forward toward my demise, my knee uncontrollably driving straight into Leo’s snarling hip.
“Ooooffggh! Ow!! What are you DOing!?!?”
My mitted mitts caught on his beefy shoulder and pushing off, I sprang from the bed, tore open the window, and threw up my snow-suited-seat onto the sill.
“Stop! Hey!! Knock it off. It’s cold out there!!!”
Despite the suggestion, I didn’t make conversation and continued to clumsily stuff my fluff filled nylon encasing through the portal, back-first, fending off the attacks with my big burgundies. As I toppled head down and backwards into the huge pile of snow on the roof, the window slammed closed behind me.
Silence. Only a passing yen, ‘Uh oh…hadn’t thought that part through…hope he lets me back in.’ I brushed it off because, for now, staring up at the moon in the crystal-clear bitter night air, stars twinkling in the black void of space and faint visions of the emerald & seafoam glow of the Aurora Borealis dancing far above, I was right where I wanted to be.
I was ready. …ready for my wondering eyes to catch a glimpse of a miniature sleigh and eight tiny reindeer.
Key Takeaways
- The Critical Role of Vacuum Systems: Vacuum technology is essential in freeze-drying processes, enabling the sublimation of ice directly into vapor, which is crucial for preserving product integrity.
- Importance of Precise Air Pressure Control:Maintaining specific pressure levels is vital to ensure efficient sublimation and prevent product degradation during the freeze-drying cycle.
- Energy Efficiency Considerations: Optimizing vacuum system performance can lead to significant energy savings, making the freeze-drying process more sustainable and cost-effective.
- Cold Traps are Essential for Vapor Management: In freeze-drying systems, cold traps play a crucial role by removing water vapor from the gas stream before it reaches the vacuum pump. This process prevents potential damage to the pump and enhances overall system efficiency.
- Compression Ratios Impact System Design: Achieving the low pressure ratios required for effective freeze-drying necessitates significant compression ratios. Understanding and managing these ratios is vital to ensure optimal performance and longevity of the vacuum system.
Freeze Drying & the Sublimation of Ice Water
The phrase pairing the two words ‘freeze’ and ‘dry’ is one of the more straightforward and clear descriptions of a process in industry. To freeze dry a blueberry, an ice cream sandwich, the latest-greatest-pharmaceutical or a formaldehyde-infused complex carbon chain; one must first freeze the liquid water (or other liquid compound) into solid ice that is integrally bound within the food or other moist substance.
Next, the material must be dried. That is, the constituent part of the thing must have all the moisture removed from it, just as if blow drying a freshly showered mane. The tricky part is, of course, that the “moisture” is now embedded within the substrate as a frozen ice brick, and it must remain coldly solid even while it is being dried away from the remaining core stuff in order to preserve the molecular pith of the desired final product.
It turns out, to freeze dry anything at all, despite the straightforward word pairing, is pretty darn difficult to pull off.
We view the molecular compound which combines two parts hydrogen to one part oxygen (H2O) as either solid or liquid since those are the two states in which our eyeballs allow us to see ice or water, respectively. Rarely do we consider transparent and ethereal steam, the vapor phase of H2O, as being equally prevalent in our lives.
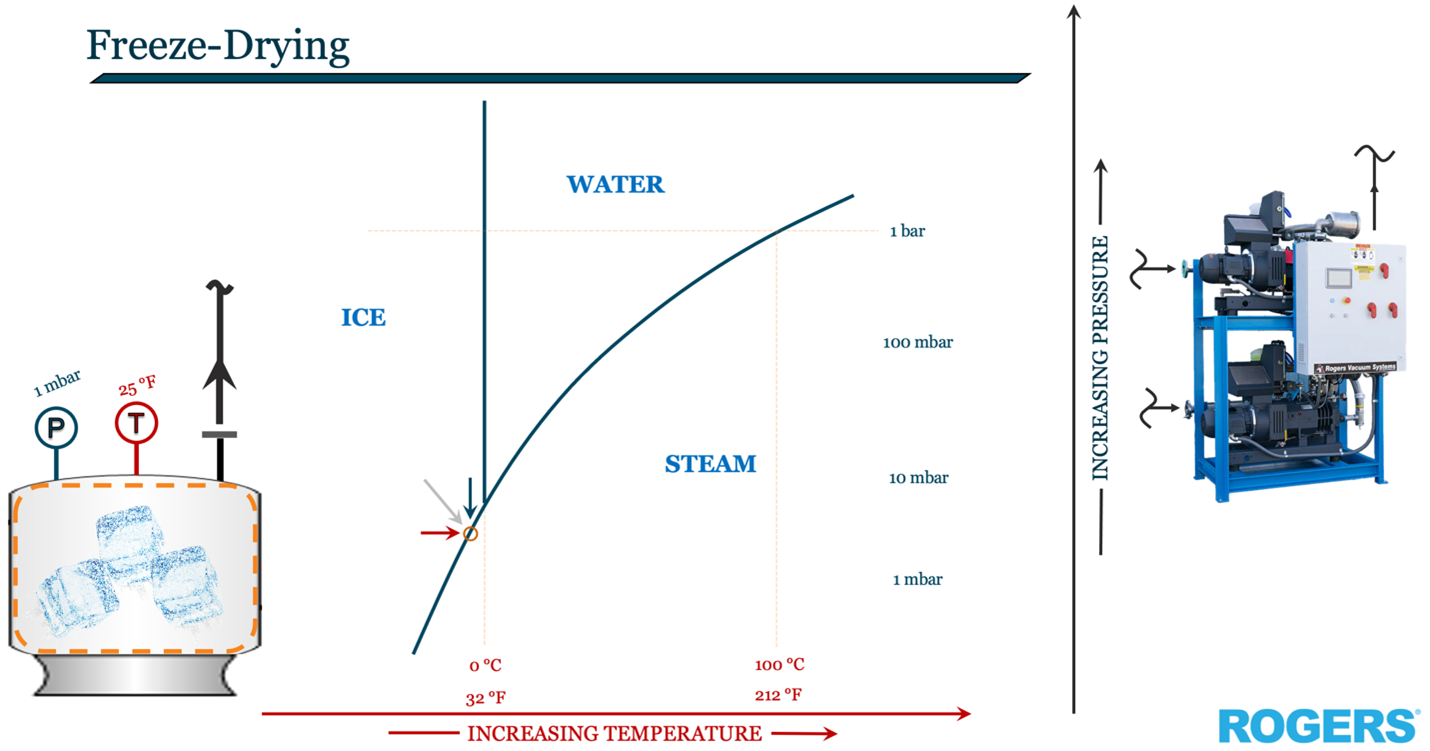
The key to freeze drying is sublimation (sub-ligh-MAY-shun). Sublimation is the phase change of a solid directly into a gas vapor without going through the liquid phase. The key to sublimation is pressure; or more aptly, the lack of pressure.
Let's take the case of water. Sublimating ice directly to steam takes very low pressure, usually somewhere on the order of a one-thousandth of an atmosphere (~1 mbar) or lower.
The phrase “low pressure” is relative. If you compare our Earth's pressure to that in the universe, the same “low” pressure of 1 mbar is infinitely high.
Observing Hot Ice in a Vacuum
Speaking of seeing is believing, human eyesight is pretty fantastic. We can see things that are very far away, particularly if nothing is in the way and those things are physically big. If you’ve ever looked up into the night sky and seen a comet (not the flying reindeer) passing overhead, you’ve been eyewitness to the sublimation of water ice occurring in the near perfect vacuum of space.

As the comet approaches the sun, the solid ice embedded within vaporizes directly into a gas. This violently explodes pockets of rock and debris. The frigid steam molecules remain suspended, bound to the comet’s core, just as our atmosphere remains gravitationally bound to Earth.
As the comet eventually leaves the inner solar system, the H2O deposits back into solid ice until the comet’s next swing through our neighborhood.
Clouds: Earth’s Coma
Bring it in a ways to back home and just about 30 miles from where you currently sit on our shared watery rock and these same basic thermodynamic processes are occurring, even right now, while you read this article. If you could drive the Griswold family station wagon there, it’d take you less than half an hour to arrive to the stratopause, a region of our atmosphere where aerosolized water droplets (we call these ‘clouds’) cannot exist.
Clouds cannot exist here because the atmospheric pressure has fallen too low while the temperature has increased some 90 above the temperature at which commercial airplanes fly. The clouds, in effect, have been freeze-dried.
Keep on going another 20 miles or so and you’d reach the upper portion of our mesosphere. By this point, the pressure has fallen further, from about 1 mbar to 0.01 mbar while the temperature has fallen again too, dropping dramatically to around -100°.
Way up here when the conditions are right, we will sometimes see clouds materialize again. Here, the clouds are not made up of liquid water droplets like the more familiar cumulus, stratus, and cirrus clouds of our lower atmosphere but instead of deposited ice crystals called noctilucent clouds.

No manmade airplane or weather balloon could ever get you there, but Santa’s sleigh might be able to take you over the solid wisps and through the mesosphere to just a little further up you go. Now in the thermosphere, you’d find yourself and Dancer dancing with the polar Auroras, in an area of our atmosphere named for the again spiking temperature.
There is still the tiniest bit of atmosphere left and the temperature is roasting. Any H2O molecules which might have happened to make it this high up are certainly sublimated again as superheated steam. From here, less than an hour’s drive away from home, and through the further beyond, the air is always bitterly crystal clear, if it is there at all.
Sublimation is happening all the time all around us, so freeze drying must not actually be that hard to pull off. Revising my previous statement, I say instead that the hard part of freeze drying is doing it from the cozy warmth of a factory floor or snowbank on a roof, either of which are located at the bottom of the ocean of atmosphere blanketing the globe.

I had no idea at the time, of course, but breathing the cold air into my lungs from the rooftop that Christmas Eve night, gazing up in anticipation of Rudolph and his team, I was peering straight through nature's blueprint for an industrial freeze dryer's vacuum system.
Vacuum Chamber to Pump: Decreasing Pressure with Undulating Temperature
Let’s put some frozen cranberries on a tray and place them in a vacuum chamber. Once the cran-ice starts to sublimate when we bring down the chamber pressure to 1 mbar; the ice will begin to get even colder than frozen. This is because, just like a pot of water boiling on the stovetop, it takes sustained heat energy to drive the sublimating phase change.
So, our freeze-drying vacuum chamber also needs some heaters to be just like our atmosphere’s stratopause, low atmospheric pressure of 1 mbar with sustained temperatures just below freezing such that clouds cannot exist, some 30 miles above.
Water expands by a factor of about 1600 times when it changes phases from a liquid or solid into a gas at standard atmospheric pressure. But we are not at atmospheric pressure inside this chamber. Instead, recall that we’re at just 1/1000th of that. Therefore, the cranberry’s embedded ice is expanding by a factor of at least 1.6 million, even by a multiplication factor more still if the chamber is operating at a lower pressure of a half or even tenths of a mbar, as many do.

This leads us to two realities. One, we must carefully control the rate of heating so that we don’t sublimate too hard and explode the cranberry as if it were a comet doing its swan dive at the sun’s inferno. We are going for a very gentle frozen simmer, as opposed to a rolling boil.
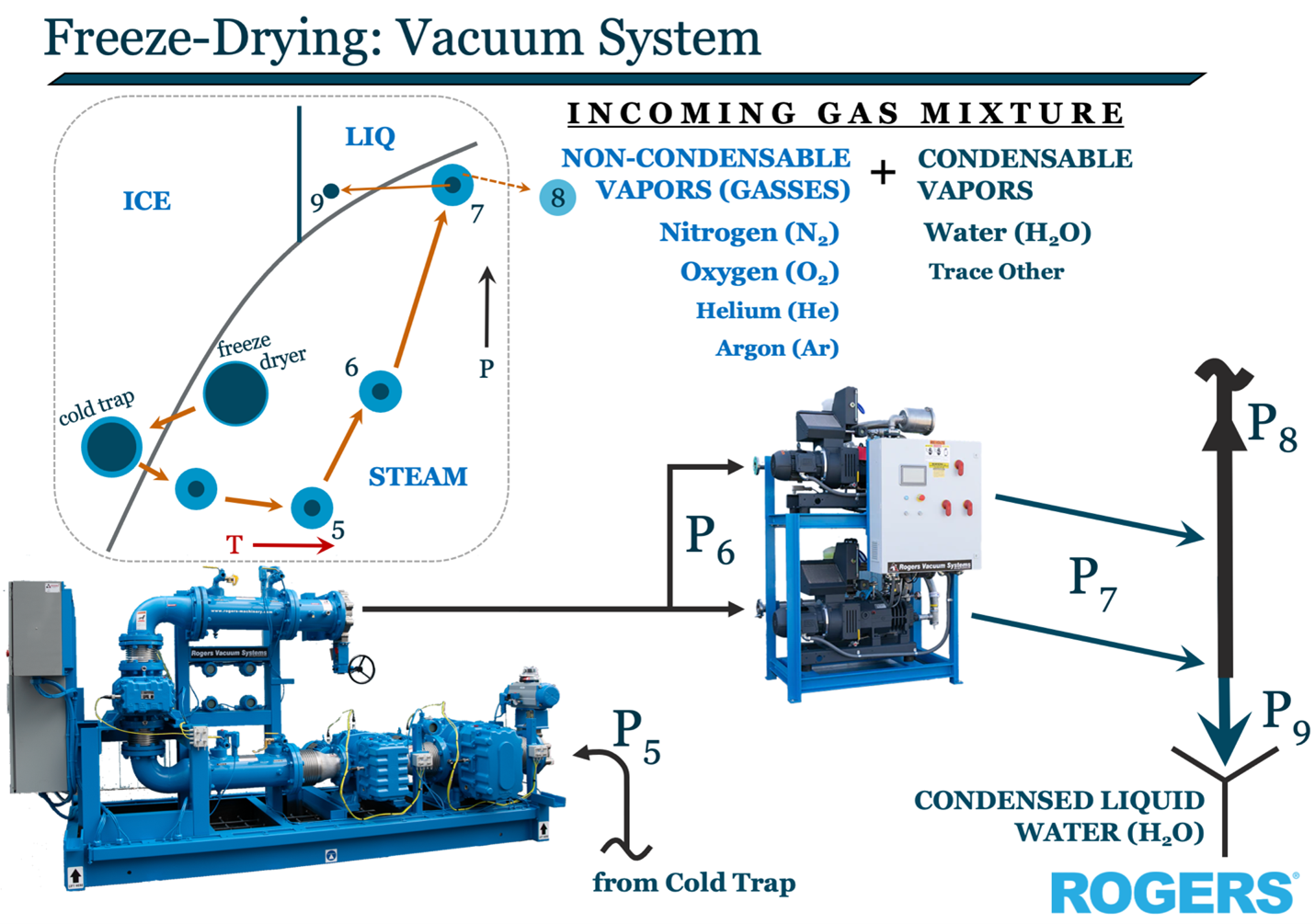
Two, we absolutely need to re-deposit the sublimated ice back out of the gas stream prior to the inlet of the vacuum pumping system if we want its relatively limited volumetric pumping capacity to have a snowball’s chance in keeping up with the onslaught of cold steam.
For this second reason and just like those noctilucent crystalline ice clouds depositing 50 miles away in the mesosphere at -100°, we must install a “cold trap” in our freeze dryer. Commonly supplied in pairs to allow for longer batch cycles, these ammonia-based refrigeration evaporators alternate back and forth, similar to the operation of a dual-tower desiccant compressed air dryer.
While one cold trap is on-line depositing ice crystals out of the vapor stream, the other is offline and warmed at atmospheric pressure, ice melting to water to be drained away from the system.
In the context of the industrial vacuum system in its entirety, the cold trap can itself be considered a vacuum source, just one that uses thermodynamics in lieu of mechanical pumping. In fact, the collapsing vapor as it is deposited like hoar frost against the evaporator tubes is the single most important aspect regarding performance of the vacuum system at large. It also has huge ramifications on the maintenance requirements of the mechanical systems yet to come downstream.
Not only does the cold trap reduce the volume of gas that the mechanical vacuum system must deal with by the same ~1.6MM:1 ratio, but it also eliminates a huge majority of what would become liquid water either inside of, or in the exhaust system, of the vacuum pump.

The relative high pressure of <1 mbar in the cold trap’s -100° pushes us along on our continuing journey to the very lowest pressure we’ll see in our vacuum system, that at the inlet throat of the mechanical vacuum pump. Or, in the case of a production operation, the first in, potentially, a series of rotary lobe vacuum boosters.
The temperature begins to naturally rise again just by the mere fact that the system piping is passing through a scorching +68° room temperature environment. This is just like when Donner, Comet (yes the flying reindeer) & Blitzen were dragging us up to the roasting thermosphere and into the beyond crystal clear resulting from the further decreasing atmospheric pressure combined with the increasing temperature.
Vacuum Systems & Condensable Vapor Load: Temperature Control
We’ll need that clear air because here comes the point of the journey where we pull our heads out of the clouds and remember the hard part, that we’re still actually sitting on that factory floor at the bottom of the ocean of atmosphere above.
Our vacuum pumping system must now do the heavy lifting of compressing the sum total of, the non-condensable nitrogen and oxygen which may have leaked in from the world, the helium or argon which may have been purposefully added for product quality and control plus any of the condensable water vapor which flew by the cold trap (there’s-never-anything-which-is-100%-efficient), to back up to just slightly above local atmospheric pressure.
Mechanical vacuum boosters & pumps are happiest compressing vapor only, as opposed to liquids and especially (duh) solids. Once we compress our resulting gas mixture to around 1,013+ mbar, any of the condensable vapors will want to do just that, condense, unless we keep them nice and hot.
At a pressure of around 0.5 mbar at the vacuum inlet throat, the required compression ratio across the vacuum pumping system will be around 2000:1. If just 3-tenths of a mbar less at 0.2 mbar, then the CR more than doubles to 5000:1. Thankfully, if there’s one byproduct that these magnitudes of compression ratios translate to, it is heat energy.
In the case of typical operating pressures and cold trap deposition efficiencies combined with a fully dry-running vacuum system, there should be ample heat-of-compression energy available to control the vacuum pump cooling such that the targeted exhaust temperatures remain high enough to maintain the vapor phase throughout the pump, into the exhaust system and down and away from the vacuum pump’s discharge port.
Lubricant or water-sealed mechanical backing pump technologies may also be used, though each present their unique application challenges, since their operating temperatures must be maintained significantly lower than their dry counterparts. Addition of inert sweep gas or ambient ballast air may be necessary to clear the pump internals and exhaust system of condensing liquids. In any case and to maximize pump life and minimize maintenance costs, it is likely desired to add such gas packages for proper start-up, control and/or shutdown procedures, as well.
Lessons In Lyophilization
Freeze dry systems are some of the more thermodynamically active vacuum applications around, with operating pressure requirements firmly categorized in the “medium vacuum” realm. The lessons learned from the principles and subsystems in play can and should be applied broadly; to your own industrial vacuum system whether freeze-drying is your game or not; and, just maybe, in appreciation of the happenstance of our own humanity.
So, this Holiday Season, just after Home Alone and Christmas Vacation but before you read the kiddos ’Twas the Night Before…, go on and head outside into the cold dark night air, look up for a miniature sleigh, and appreciate for a moment, just how fortunate we all are to be cozy and warm under our blanket of atmosphere, zooming around on this humongous and insignificant watery rock, together.

Learn more about process vacuum and oil-free technology in this article.
Learn more about our Engineered System Solutions team.
Meet the Author
 |
Bryan A. Jensen is a mechanical & aeronautical engineer with over 20 years of application experience in a wide variety of industrial manufacturing processes, focusing on the compressed air & gas industry. Beginning his career as a NASA materials research engineer, he currently heads the Engineered System Solutions team at Rogers. |





